Ancient electricity
Dendera
light
The Egyptians had some form of understanding electric phenomena from
observing lightning and interacting with electric fish (such as
the Malapterurus electricus) or other animals (such as electric
eels). The comment about lightning appears to come from a misunderstanding of a
text referring to "high poles covered with copper plates" to argue
this but Dr. Bolko Stern has written in detail explaining why the copper
covered tops of poles (which were lower than the associated pylons) do not
relate to electricity or lightning, pointing out that no evidence of anything
used to manipulate electricity had been found in Egypt and that this was a
magical and not a technical installation.
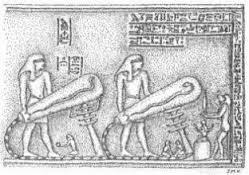
The single representation of the image, called the
"Dendera light" by some alternative suggestions, exists on the left
wall of the right wing in one of the crypts of the Hathor temple.
Those exploring fringe theories of ancient technology have suggested that there were electric lights used in Ancient Egypt. Engineers have constructed a working model based on their interpretation of a relief found in the Hathor temple at the Dendera Temple complex. Authors (such as Peter Krassa and Reinhard Habeck) have produced a basic theory of the device's operation. The standard explanation, however, for the Dendera light, which comprises three stone reliefs (one single and a double representation) is that the depicted image represents a lotus leaf and flower from which a sacred snake is spawned in accordance with Egyptian mythological beliefs. This sacred snake sometimes is identified as the Milky Way (the snake) in the night sky (the leaf, lotus, or "bulb") that became identified with Hathor because of her similar association in creation.
Baghdad Battery
Baghdad Battery, sometimes referred to
as the Parthian Battery, is the common name for a number of artifacts created
in Mesopotamia, during the dynasties of Parthian or Sassanid period
(the early centuries AD), and probably discovered in 1936 in the village
of Khuyut Rabbou'a, near Baghdad, Iraq. These artifacts came to wider
attention in 1938 when Wilhelm König, the German director of the National
Museum of Iraq, found the objects in the museum's collections. In 1940, König
published a paper speculating that they may have been galvanic cells,
perhaps used for electroplating gold ontosilver objects. Though
far from settled, this interpretation continues to be considered as at least a
hypothetical possibility. If correct, the artifacts would predate Alessandro
Volta's 1800 invention of the electrochemical cell by more than a
millennium.
Description and
dating
The artifacts consist of terracotta pots
approximately 130 mm (5 in) tall (with a one-and-a-half-inch mouth)
containing a copper cylinder made of a rolled-up copper sheet,
which houses a single iron or (galvanized nail) rod. At the top, the iron
rod is isolated from the copper by bitumen plugs or stoppers, and
both rod and cylinder fit snugly inside the opening of the jar, which bulges
outward toward the middle. The copper cylinder is not watertight, so if the jar
were filled with a liquid, this would surround the iron rod as well. The
artifact had been exposed to the weather and had suffered corrosion, although
mild given the presence of an electrochemical couple. This has led some to
believe that wine, lemon juice, grape juice, or vinegar was
used as an acidic electrolyte solution to generate an electric
current from the difference between the electrochemical potentials of
the copper and iron electrodes.
König thought the objects might date to the Parthian period (between 250 BC and AD 224). However, according to St John Simpson of the Near Eastern department of the British Museum, their original excavation and context were not well-recorded, so evidence for this date range is very weak. Furthermore, the style of the pottery is Sassanid (224-640).
Most of the components of the objects are not particularly
amenable to advanced dating methods. The ceramic pots could be analysed
by thermoluminescence dating, but this has not yet been done; in any case,
it would only date the firing of the pots, which is not necessarily the same as
when the complete artifact was assembled. Another possibility would be ion diffusion analysis,
which could indicate how long the objects were buried.
Electrical
Copper and iron form an electrochemical couple, so that, in
the presence of any electrolyte, an electric potential (voltage)
will be produced. This is not a very efficient battery as gas is evolved at an
electrode, the bubbles forming a partial insulation of the electrode so that
although several volts can be produced in theory by connecting them in series,
their internal resistance from the formation of the gas bubbles becomes so
great that it severely limits the electrical current that can be
produced from such a simple wet cell.
König had observed a number of very fine silver objects from
ancient Iraq that were plated with very thin layers of gold, and speculated
that they were electroplated using batteries with these as
the cells. After the Second World War, Willard Gray demonstrated current production
by a reconstruction of the inferred battery design when filled with grape
juice. W. Jansen experimented with benzoquinone (some beetles produce quinones)
and vinegar in a cell and got satisfactory performance.
However, even among those believing the artifacts to be
electrical devices, electroplating as a use is not well-regarded today. Paul
Craddock of the British Museum said "The examples we see from this region
and era are conventional gold plating and mercury gilding. There’s never been
any untouchable evidence to support the electroplating theory." The gilded
objects that König thought might be electroplated are now believed to have
been fire-gilded (with mercury). Reproduction experiments of
electroplating by Arne Eggebrecht consumed "many" reproduction cells
to achieve a plated layer just one micrometre thick. Other scientists noted
that Eggebrecht used a more efficient, modern electrolyte; using only vinegar,
the battery is very feeble.
Source : Wikipedia
Source : Wikipedia





.jpg)
Comments
Post a Comment